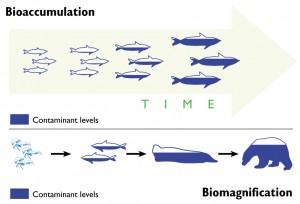
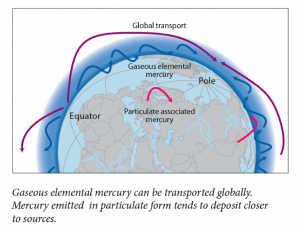
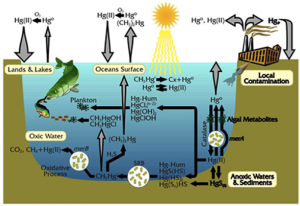
This is a guest blog from Lawrence Susskind, Ford Professor of Urban and Environmental Planning at the Massachusetts Institute of Technology and Visiting Professor at Harvard Law School. Professor Susskind specializes in global environmental treaty making and the mediation of public disputes. In this post, reprinted with his permission from his blog “The Consensus Building Approach,” he explains some key lessons for good negotiation, including how to get to a positive overall outcome without compromising your personal (or your nation’s, in the case of INC5) interests. As we near the final hours of the mercury treaty negotiations, we’ll be watching the delegates’ negotiation techniques and hope to see some of Professor Susskind’s advice at play out on the discussion floor.
by Lawrence Susskind, reprinted from The Consensus Building Approach
I hear the phrase “win-win” all the time. I’m not sure that very many people who use it know what they are talking about. I have a hunch they mistakenly assume that if everyone would just cooperate, then all parties would get what they want. That, of course, is ridiculous. There are almost no negotiations in which everyone can get everything they want. And cooperation or even compromise isn’t the issue.
Thinking Clearly About Win-Win
No one should agree to anything in a negotiation that is worse for them than what they are likely to get if no deal is reached. Roger Fisher and Bill Ury made this point thirty years ago in Getting To Yes. First, figure out what no agreement is most likely going to leave you with, try to generate something (a walk-away) that’s better than that, but when you are in an actual negotiation don’t reject something that’s better than your realistic walk-away, even if it won’t get you everything you’d like to have. Fisher and Ury called this point of comparison, your Best Alternative to the Negotiated Agreement (BATNA). A win-win negotiation is something that gets all sides an outcome better than their BATNA. It doesn’t necessarily get anyone everything that they might want.
Decision analysts (like Howard Raiffa) talk about the same idea in terms of a negotiator’s Reservation Price — the amount that they’ve decided ahead of time they won’t accept “less than” or “pay more than.” Putting aside for a moment that BATNAs and Reservation Prices are sometimes hard to estimate or “know” ahead of time, a win-win negotiation can be thought of as a deal in which all sides “gain” relative to their best estimates of their walk-aways.
Entering the Trading Zone
As many negotiation experts have explained, the beginning of a negotiation is hard because the parties all think they know what they want, all think they know what the other side(s) want and have all worked through with their own constituents what they will and won’t accept. If they then spend all their time pushing their own objectives, sometimes even exaggerating “what they have to have,” and giving arguments in anticipation of what others will say, they probably won’t get a deal. On the other hand, they have no choice but to show their “people” that they are trying hard to be victorious. So, there is a lot of wasted motion, a lot of exaggeration and a lot that’s done for show. At some point, though, usually behind-the-scenes, each party has to re-assess. “Am I going to be able to get something equal to or better than my walk-away.” “I know I can’t get everything I have demanded, but a lot of that was made up anyway.” Most people think that in a bargaining situation, you have to ask for more than what you really want so that you can make concessions and still end up with your real goal. Of course, that strategy can backfire. If your constituency hears you make outrageous opening demands and then you don’t “bring home the bacon,” it may not be possible to back down from those demands without losing face. The fact is, that if everyone were being completely honest about their most important interests (i.e. the things that are important to them in rank order), and they all felt comfortable talking about these items, the negotiators could then engage in productive joint problem-solving to see what sort of trades might (or might not) permit all parties to meet or exceed their realistic walk-aways.
If the opening of a negotiation doesn’t alienate all the parties (because one or more sides has taken an absolutely outrageous stand for tactical reasons and did so in an obnoxious way), they can then enter the “trading zone.” This is the negotiation space in which parties try out various new ideas and possible trades. “Now that I’ve heard what’s really important to you, what if I gave you X, would you give me Y?” Those kinds of linked offers are the key to creating value. If I have something you want very much and it’s not that important to me, and you have something I covet, and it is not crucial to you, when we trade those two things, that creates value. That’s not compromise. We can only do this, however, if we can find our way into the trading zone. Fisher and Ury point out that parties have to be willing to engaging in “inventing without committing” for this to happen. There are other procedures that can also be used to make this work. Once in the trading zone, though, the parties have to do all they can to explore numerous “what-if’s?” to see if they can create value. Then, once they have created all the value they possibly can, the parties need to go back and see if they can put together a “package” that ensures everyone something above their BATNA or their Reservation Price. The process gets more complicated when values or rights are involved and not just interests, but the same Mutual Gains Approach (MGA) can be used even in those situations.
What If the Parties Haven’t Done Their Homework or Aren’t Authorized to Make a “Reasonable” Deal?
The key to win-win negotiation is not compromise, it is getting into the trading zone and creating as much value as possible. If all negotiations involved just two parties and those negotiators didn’t have to report to anyone, the process would not be that difficult. But, most of the time, negotiators have someone else (often a diverse and fractious constituency) to whom they must report, and to whom they are accountable. This makes moving into the trading zone more difficult.
Think about negotiators who represent their county in a multinational treaty negotiation. Each negotiator spends months talking with different agencies and political actors inside their country, trying to reach a delicate balance on what to stress and what to sacrifice when formal multi-country negotiations begin. Then, when the negotiators sit across from each other in the big hall, each reads the script that they worked out so carefully back home. It doesn’t matter if the formal statements that each negotiator gives appear to ignore what previous speakers in the big hall have said. The fact is, that’s exactly what’s happening. The negotiator is playing to his or her home crowd. Any deviation from the pre-prepared script would probably be cause to drag the negotiator home and demand their resignation. But, at night, at the bar, when the country negotiators chat informally, new packages emerge, and new trades are explored. As the end of the two week formal negotiation period is about to draw to a close, the chair of the session hands out a revised version of the treaty text. This is quite different from the one that countries spent the previous six months reviewing. That earlier version was what they considered when they helped to write their opening speech for the big hall. Now, however, the negotiator has to call “home.” (And, in each country home is represented by a different political figure or set of actors.) They have a choice to make (and almost no time to make it). “Do we support the revised version of the treaty that the chair has sprung on the assembly at the last minute?” Yes, or no? There’s no time for further revisions, everyone has return flights scheduled in a few hours. Moreover, in an international treaty negotiation, the chair won’t ask the parties to vote. Rather, he or she will ask, “Do I hear consensus? Do you support the revised version of the proposed treaty?” It is entirely up to the chair whether he or she “hears” consensus. At the point, the country negotiators have the option of signing the document on their way out their door. Then, each negotiator has to go back to their home legislature and seek ratification (in America’s case by a vote of the United States Senate).
Think of the poor negotiator for a country that didn’t do its homework ahead of time, or didn’t give it’s negotiator any room to maneuver. They can’t have much impact on the final outcome because they can’t participate in the discussion of informal trades. Ideally, a negotiator needs to know what his or her country’s most important interests are (and which of many ways of meeting them will be OK). The negotiator may also have instructions to take a strong opening stand, but at the same time be empowered to use whatever informal channels are open to enter the informal trading zone and see what new options can be created. Until the final moments, the negotiator is just exploring “what-if’s.” When the chair produces the final draft of the proposed treaty, the negotiator needs to know who they can call to get a yes or a no (although they must be able to report that they have found a way to ensure that their country’s most important interests have been met).
How Much Can and Should You Do for the Other Side?
There’s nothing more frustrating than trying to negotiate with someone who isn’t prepared (i.e. doesn’t know what their group’s interests really are), isn’t authorized to enter into informal discussions about ways of creating value, and isn’t empowered to commit to anything other than what their group discussed before the negotiations began. If you are sitting at the table (in the big hall) with negotiators who are in one of these three positions, what can and should you do? First, even though it is awfully late in the game, you might encourage the other negotiator to be in touch with his or her group to clarify what their key interests really are. This should take the form of a BATNA or walk-away analysis. (“What are we going to be left with, realistically, if there is no agreement?” Not, what will we demand?) This will help the negotiator avoid the bad mistake of turning down “pretty good” offers. Second, you can construct several “alternative packages” for the negotiator to bring back (quickly) to his or her group. Each should spell out why and how a package will help the group meets in most important interests, and at what cost and with what risks. Third, you can coach the other negotiator a bit, teasing out for them what they might say to their constituents in response to various criticisms or complaints. Of course, if the negotiation is being managed by a neutral party (a mediator or a facilitator), then any participant can have a private conversation with that individual without having to reveal to any other parties how unprepared they really are (or how mixed up their constituency might be).
So, When Should You Compromise?
No negotiating party should ever accept an agreement that is worse for them than no agreement. But, a group may be very uncertain about how to predict what no agreement really means. (“If we don’t reach agreement now, what will happen next and what effects will that have?”) So, they might say yes to something that turns out, in retrospect, to be less desirable for them that having said no. But, no negotiator should ever agree to something that knowingly “hurts” their constituency (i.e is worse than what no agreement held in store for them) just to be liked. Good working relationships (and particularly trust) are not achieved by caving in to pressure. Rather, they are a by-product of all sides acting in what Fisher and Ury would call a “principled way.” And, I would argue that one principle of negotiation is that no one should ever “give away” their interests in the hope of “buying” a good relationship. All that will do, as Fisher and Ury point out, is teach the other parties that the same behavior be expected in the future.
“Good for You, Great for Me”
So, in actuality, a win-win outcome is one that gets all parties more than what no agreement would have guaranteed them. But, that doesn’t mean that all parties “gain the same amount.” I might like an agreement because it get’s me well above my BATNA. You might grudgingly accept my proposed agreement because it gets you more than what you are likely to get if we reach no agreement at all. Win-win agreements do no promise all sides equal or similar gains. They only promise that all sides — because they enter into the trading zone, engage in joint problem-solving, and agree to be realistic, even honest, about their highest priority interests — get an outcome that is better than their most realistic estimate of what they would have ended up with had they walked away with no agreement.
Thus, the way to “win” at “win-win” negotiation is to make sure that you come up with a proposed agreement that is “good” for other side(s) and “great” for you. You can only do this by working hard to uncover and respond to the most important interests of the other parties. Whatever “opening” stand you take (to ensure your “people” that you are fighting hard on their behalf), you have to be able to move from there into the trading zone and function effectively in that “what-iffing” environment. Then, you must have the right mandate from your “side.” That is, you need to have worked out ahead of time a clear understanding of your group’s priority interests. And, you need to know who you can call for authorization to enter into an anticipated agreement at the last minute as long as the package exceeds your group’s realistic estimate of what no agreement means to them.