MIT Mercury team, Saturday morning, tired but celebrating the negotiation conclusion! L-R: Ellen Czaika, Philip Wolfe, Bethanie Edwards, Amanda Giang, Julie van der Hoop, Mark Staples, Noelle Selin
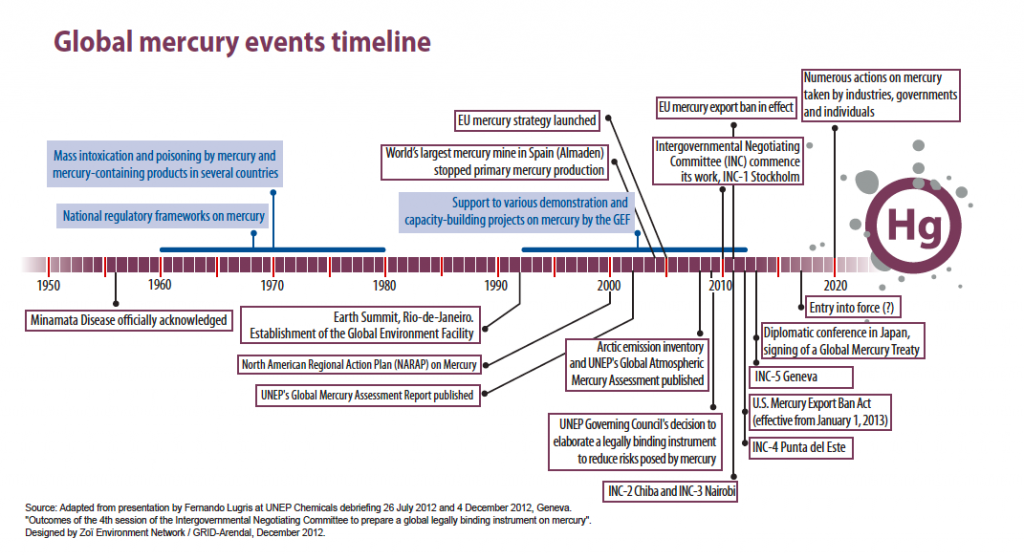
Early in the morning, at 7 AM on Saturday, January 19 in Geneva, 140 countries agreed to adopt a new, global treaty on mercury. The Convention will be signed in early October in Kumamoto, Japan, and will be called the Minimata Convention, in recognition of the city in which methylmercury poisoning was identified in the 1950s.
Over the past week, we’ve covered many of the outstanding issues in the negotiations. In this post, I’ll give a brief roundup of some of the major areas of agreement. Over the next week, the teams that covered each issue will provide some more detail about the specifics in the final agreement. In addition, we’ll all be posting our individual reflections on the events of the week.
Mercury Supply, Trade and Waste
The major agreement reached in the area of mercury supply is that countries must eliminate existing primary mercury mining fifteen years after becoming a party to the Convention. New primary mining is not allowed. During the fifteen-year transition period, mercury from primary mining can only be used in certain products and processes allowed by the Convention.
A prior informed consent procedure also applies to the export of mercury.Parties must manage mercury waste in an environmentally sound manner, taking into account the guidelines of the Basel Convention. See our earlier overview of supply and trade and waste issues for more background on these issues.
Products and Processes
The Convention phases out the manufacture, import and export of several mercury-added products by 2020. Examples include thermometers and barometers, cosmetics with mercury content above 1 part per million including skin lightening soaps, pesticides, certain lamps with high mercury content, and most mercury-containing batteries. The use of mercury-containing dental amalgam is to be phased down, with no particular date attached.
For processes, the use of mercury in chlor-alkali production is to be phased out by 2025, and in acetaldehyde production by 2018. Parties to the Convention may request exemptions from these requirements for an additional five years, with exemptions renewable once by the Conference of the Parties. For more background on products and processes, see the guest post by Hannah Horowitz on mercury in unexpected products and our background post.
Artisanal and Small-Scale Gold Mining (ASGM)
Parties with artisanal and small-scale gold mining (ASGM) are required to reduce, and where feasible eliminate, the use of mercury and releases to the environment from this activity. National plans to address this issue are required for parties that have significant ASGM mercury use. For more on the concerns about ASGM, see our issue overview.
Health
The Minimata Convention has a dedicated section devoted to health aspects, which negotiators referred to as precedent-setting for a multilateral environmental agreement. The section encourages the promotion of activities to help populations at risk of adverse mercury health effects, and the cooperation and exchange of information with the World Health Organization, the International Labour Organization and other intergovernmental organizations. For more on the health effects of mercury, see our earlier post.
Emissions and Releases
The agreement has similar but distinct requirements for mercury emissions to air versus releases to land and water. In both cases, Parties may develop national goals, must prepare inventories, and must take some measures of control. For air emissions, the treaty identifies relevant sources as: coal-fired plants and boilers, non-ferrous metal mining activities, waste incineration, and cement production.
The agreement distinguishes between new and existing emissions sources. For new relevant sources, Parties must apply Best Available Techniques (BAT) and Best Environmental Practices (BEP) within five years of the Convention’s entry into force. For existing sources to air, parties have ten years to act, and can choose between applying goals, emissions limits, BAT/BEP, multi-pollutant control options, and other measures that reduce mercury. A similar menu of options is available for releases to land and water. Earlier, we covered the technologies that are available to control mercury emissions and some of their co-benefits and also wrote an issue overview.
Financial Mechanism
The financial mechanism for the Convention includes two elements: the Global Environment Facility (GEF) trust fund, and a specific international programme to support capacity-building and technical assistance. The GEF is to provide new resources to meet implementation costs, guided by the Conference of the Parties to the Minimata Convention. The programme will be operated by the Conference of Parties, hosted by an existing institution and consisting of voluntary contributions. For more details and background about the importance of financial and technical assistance, see our issue overview.