by Danya Rumore
The fifth meeting of the International Negotiating Committee to Prepare a Legally Binding Instrument on Mercury (INC 5) officially began yesterday on an overcast, chilly day here in Geneva, Switzerland.
The morning began with a demonstration by the International POPs Elimination Network (IPEN), an organization working for a toxics-free future, at 8:30am. Their main concern: the treaty should be called “A Global Mercury Treaty”, not “A Minamata Convention”, because this treaty may not be sufficient to prevent future mercury-related health tragedies like that experienced in Minamata, Japan.
Following IPEN’s demonstration, we spent about 15 minutes trying to find our way to the third floor balcony in the labyrinthine International Conference Center. Finally making our way through the maze of stairs, hallways, and doors to the NGOs nosebleed seats (to use another attendee’s term), we sat down to enjoy the traditional yodeling session that kicked off the day’s official events.

At 9:30am, the yodeling ceased and the plenary session began. The session started with the opening ceremony, in which the attendance of about 900 delegates from 140 countries was noted and INC Chair Lugris urged participants to focus on finding consensus. While languages from around the world were spoken on the floor, everything was translated into English (and numerous other languages) and transmitted to participants through headphones available at each seat.

Then came the delegates’ opening statements. In statements ranging from 2-20 minutes, delegates gracefully thanked the Chair for his work, expressed their appreciation for Switzerland hosting the meeting, and made clear their positions on the treaty. I think we were all somewhat amazed by how not surprised we were by nations’ and NGOs opening statements; they were more or less exactly what someone familiar with the issues on the table would expect (opening statements are detailed in the Earth Negotiations Bulletin (ENB) newsletter). However, we were a little surprised—and quite amused—by the Philippines delegate’s mention that he hoped Chair Lugris enjoyed his recent holiday in the Philippines, to which the Chair replied that he did.
Opening statements continued until a little after 1:00pm, when everyone filtered out of the stadium-style plenary room, down the maze of stairs, to the host country’s welcome lunch reception. Despite having to fight against apparently hungry delegates to get food, we enjoyed a buffet including everything from cold cuts and salads to potato soup with truffle oil. We also enjoyed live Swiss music from a band of two musicians dressed in traditional Swiss garb (one barefoot) playing zithers with a third musician, wearing a black suit, playing a bass (it was an interesting trio, but the music was excellent).

Amid the music and buffet, we mingled with delegates and other NGO representatives, talked about our poster with people passing by our table, and discussed the happenings of the morning. Then we loaded up on dessert and plenty of coffee to get us through the afternoon session, and we returned to the plenary for a discussion of the draft treaty text.
The afternoon plenary began with a discussion of the treaty’s preamble, during which delegates proposed adding a direct mention of Minamata, referencing indigenous peoples, including health impacts, invoking the precautionary principle, and bringing the polluter pays principle into the preamble’s language.
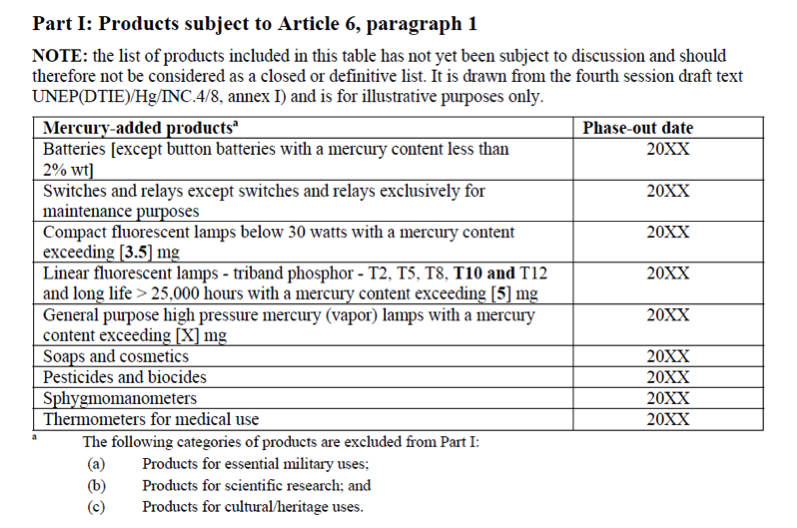
Following discussion of the preamble, the plenary moved on to the topic of products and processes (watch for Bethanie’s and Ellen’s upcoming blog on this topic). After much debate about Articles 6, 7, 8, and 8b, it was decided that a contact group would meet in the evening to continue discussion of this topic area.
Before breaking for dinner, the delegate from Saudi Arabia represented the interest of the everyone at the conference by making the statement “We seem to be having problems with the internet…” At a paperless meeting where all documents are shared over the intranet, good internet connection is a non-negotiable issue.

Following a dinner break (i.e., a time to search for outlets in order to charge our laptops), our MIT team divided into two groups: one group returned to the plenary for the discussion of financial and technical assistance and the other group went to observe the contact group discussion on products and processes.
In the contact group on products and processes, the debate about Articles 6, 7, and 8 continued, with the US and Canada largely dominating the conversation. While some progress was made before the close of the session a little after midnight (see ENB newsletter for more details), much work remains to be done on the subject of products and processes.
In the plenary, the discussion about financial and technical assistance was largely dominated by a sharp divide between 1) the nations that love the Global Environment Facility (GEF) and the nations that do not love the GEF, and 2) those that want technology transfer and those that are seemingly unwilling (or, as many developed nations put it, not able) to provide it.
The conclusion of the plenary discussion, ending late in the night, was that a contact group would meet today to continue discussion on Article 16 (technical assistance and, possibly, capacity building) but not Article 15 (financial resources and mechanisms). For now, the contentious Article 15 is on hold, and the first item on the agenda for the plenary during day 2 is emissions and releases.
So concluded Day 1 of the INC5 negotiations and, as I write this, day 2 is in full swing. Check out Ellen’s blog on “What to expect from day 2” to learn more about what’s coming up in today’s negotiations.