by Danya Rumore and Mark Staples
Danya and Mark here. During the INC5 negotiations, we’re covering issues related to mercury waste, mercury trade, and artisanal and small scale-gold mining (ASGM). We’ll be providing overviews of each of these issues separately, to make them more digestible. Here, in the first of our three Issue Overview installments, we provide an explanation of the mercury waste issue, what is already included in the draft treaty text about this issue, and what is likely to be discussed—and hopefully decided—in the week ahead.
The use of mercury in products and processes has a long history, with evidence of human use of mercury dating as far back as 5000 BCE.
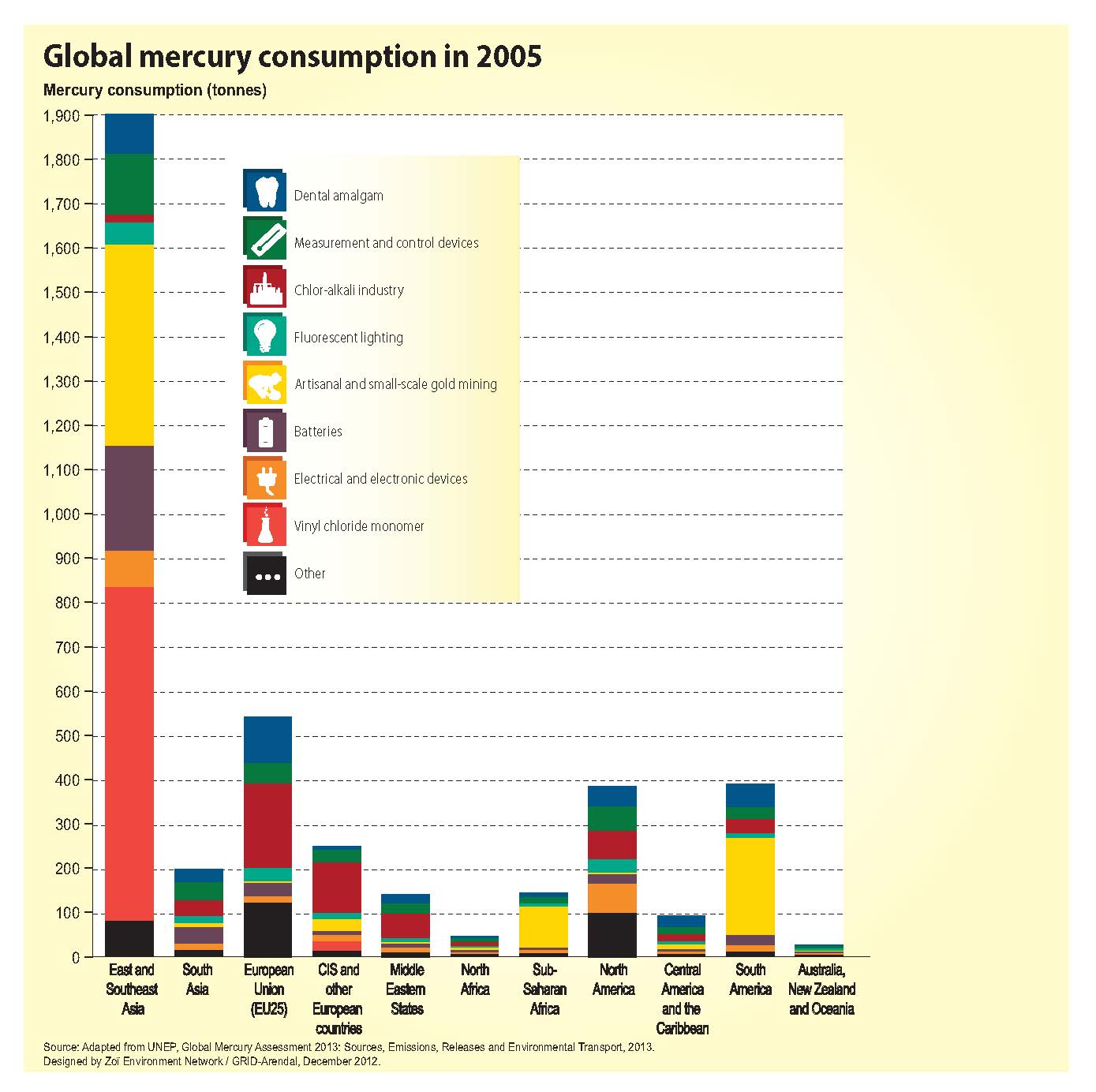
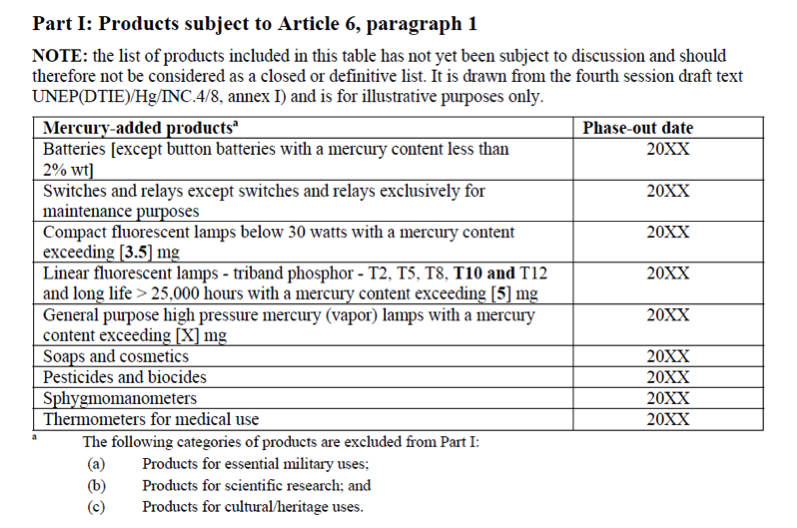
Although awareness of the health and environmental impacts of the toxic metal has resulted in reduced use of mercury in many industries, it is still present in many products and processes, including light bulbs, cosmetics, and chlor-alkili production. Many mercury-containing products eventually end up in landfills or other waste sites, and leftover mercury compounds from industrial processes often enter the waste stream. When deposited in landfills, mercury-containing waste, over time, releases mercury into the environment. More problematically, incineration and the combustion of mercury containing waste can result in a sudden and significant release of mercury directly into the atmosphere. According to the UNEP Global Mercury Assessment 2013, waste-related sources made up approximately 5% of global anthropogenic emissions in 2010.
While mercury in the waste stream is a pressing issue, the good news is that solutions are available. Controlling and reducing the use of mercury in products can prevent mercury from entering the waste stream in the first place. Since significant amounts of mercury already exist in products and waste, efforts to capture, contain, and recycle mercury-containing wastes are necessary. Such efforts are already underway, such as the US EPA’s fluorescent lamp recycling program and guidelines in case of releases and spills. Further, emissions controls on waste incinerators can greatly reduce mercury output from waste combustion and should be implemented wherever possible.
During the INC4 negotiations in Uruguay, progress was made on the question of how to address mercury waste in the globally binding agreement. Most prominently, the draft treaty text includes the provision that all parties to the agreement shall take appropriate measures to manage mercury waste in an environmentally sound way, in accord with the Basel Convention. This part of the treaty seems to be generally accepted, although the question of how to manage the transport of mercury across international boundaries in circumstances where the Basel Convention does not apply remains unresolved.
On Monday, the articles of the draft text relevant to mercury waste were introduced in the plenary session. Switzerland, with support from the EU, called for bringing all definitions and procedures for the trans-boundary movement of mercury in line with the Basel Convention. Lebanon expressed a desire for standards specific to mercury waste disposal, and Chile called for a more clear definition of “mercury wastes”. Additionally, whether and how to make parties who have not signed or ratified the Basel Convention comply with transboundary waste movement regulations was discussed.
The draft treaty also includes text related to the identification and management of sites contaminated by mercury. This topic appears to be much more contentious than the topic of waste management. While the draft treaty includes language indicating that action shall be taken to reduce the risk presented by contaminated sites, it remains to be seen whether capacity building and financial and technical assistance will be a necessary condition of including this in the agreement. In the plenary, Japan requested deletion of the capacity building and assistance provision, while Brazil, Iran and Morocco called for its inclusion.
At the conclusion of the plenary session on the second day of official negotiations, the Chair elected to move discussions of the treaty articles on storage, waste, and contaminated sites to the contact group for selected technical articles. Before addressing these issues, the contact group must first work through the products and processes articles. For now, storage, waste, and contaminated sites are on hold. We anticipate they will be picked up again late Tuesday evening or, more likely, early in the day on Wednesday.
As developments emerge, we’ll be posting updates here on our blog and via twitter @markdstaples and @DanyaRumore. Stay tuned!