by Alice Alpert, Ellen Czaika, and Amanda Giang
Pathways to exposure
Although these negotiations are explicitly focused on creating an environmental treaty, mercury’s major significance is its toxicity to humans. When you think about mercury, you probably picture a mercury thermometer. In a thermometer, you can literally see the silvery mercury in its bulb – this is liquid, elemental mercury. If you are absent minded and accidentally drop that mercury thermometer on the bathroom floor, the mercury will spills and form into beads. Although it’s not a good idea to touch this mercury, it is also not easily absorbed by the digestive system in this form.
The more pernicious way for this mercury to enter your body is if it vaporizes, which happens to a small amount of the liquid mercury at room temperature. If you inhale the vapor it can easily pass from your lungs into your blood stream and damage tissues. In fact, vacuuming up the spilled mercury can increase its vaporization and therefore the danger.
In truth, most people will not be exposed to mercury in this form. Instead, people working in chlor-alkali production, mercury mining and refining, thermometer production, dentistry, and in the production of mercury-based chemicals are at increased risk. Although measures have been taken to limit occupational exposure to mercury, many workers may continue to be at risk. Similarly, artisanal or small-scale gold miners are routinely exposed to mercury vapor at very high levels, in the process of burning the mercury-gold amalgam used to extract gold from ore. Indeed, miners and their communities often exhibit clear signs of mercury poisoning.
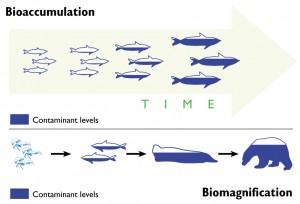
Another important pathway for mercury exposure is through eating seafood. In fact, according to the World Health Organization (WHO) (Section 2.4, paragraph 128), for many people this is the main pathway for human exposure to methylmercury. Exposure happens through the process of bioaccumulation and biomagnification. In brief, mercury is methylated to methylmercury (CH3HgX) by bacteria in the ocean and then accumulates in fish and marine mammals. Long-lived predatory fish at the top of the food-chain, such as swordfish, tilefish, shark, and tuna, can accumulate dangerously high concentrations of mercury. The US EPA lists guidelines for safe consumption of fish. Women who are pregnant or who could become pregnant should be especially careful about eating mercury contaminated fish because the mercury can be harmful to the developing fetus.
In addition, exposure could happen through dental amalgams. Elemental mercury is used in dental amalgam, and it can be ingested or its vapors can be inhaled. This is a contentious issue in the negotiations. The American Dental Association and the US Environmental Protection Agency state that mercury in dental amalgam is safe, while a report by the WHO (p.11) states that dental amalgam is a significant source of mercury exposure in those who have mercury fillings. We encourage you to look into the reports if you are concerned about this issue. For a solid overview of all pathways, see the WHO report on mercury exposure.
How and why does mercury make us so sick?
The most serious effects of elemental mercury vapor concern the nervous system, including tremors, erethism (a neurological disorder characterized by irritability and shyness), insomnia, muscle weakness, and memory loss. At especially high concentrations, the kidneys, thyroid, and pulmonary system can be affected. Similarly to elemental mercury, mercury in its organic form, methylmercury, has serious neurological effects, including neurobehavioral deficits, neuronal loss, loss of muscle movement, hearing loss, paralysis, and death.
Why is mercury so toxic for the nervous system? There are two specific processes: first, elemental mercury and methylmercury can easily cross the blood-brain barrier and once in the brain, can be oxidized to the mercuric ion (Hg2+), which cannot cross back across the barrier. Instead, mercury is trapped in the brain, where the second process begins, neurotoxin by excitotoxicity. What? Okay, we’ll slow down and explain these multi-syllabic words: in studies of rats, Hg2+ inhibits glutamine and glutamate transport, causing receptors for these molecules to become overexcited. This causes a large influx of the calcium ion into the cell, which activates enzymes that can lead to the neuron’s death, and thus the serious neurological effects. This second process is the reason why mercury is so toxic.
Mercury crucially effects developing fetuses. In the same way that methylmercury can cross the blood-brain barrier, it can also pass through the placenta from a mother to her fetus and then to the developing fetus’ brain. As a neurotoxin, methylmercury can also damage its nervous system, and in fact mercury has lasting negative effects when fetuses are exposed to concentrations at levels that are only 10%-20% of toxic levels for adults.
Babies born to women who consumed significant amounts of methylmercury while pregnant display symptoms similar to cerebral palsy, including delayed walking and talking, altered muscle tone and reflexes. Tragically, these impairments are permanent and affected will suffer from these impairments for their entire life. In fact, recently published research estimates that IQ reductions due to chronic, low-level fetal mercury neurotoxicity costs the European Union alone € 8-9 billion euros per year. Clearly, there are significant social and economic impacts from mercury exposure, particularly for the young.